What Happens When an Electron Is Removed From an Atom
10 In which electron shell do electrons have the highest potential energy. Ions are still atoms but with a different electronic configuration.
13 What would happen if an electron was removed from an atom.
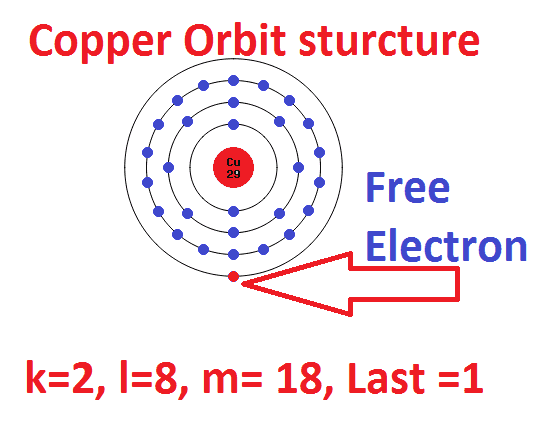
. 10 When an electron gets closer to the nucleus does it attract or repel. A hydrogen atom is commonly referred to as merely a proton since it contains just one proton and no electrons whereas a H atom has one of each. With 0 eV energy the electron loses its interaction with the atom.
12 Are valence electrons attracted to the nucleus. As Chris said when one removes an electron from the atom it is bound to one creates an ion in that case one creates a positively charged ion since the electron has a negative charge. That is there are more protons in the nucleus positive charges than there are electrons negative charges.
11 What happens as electrons get closer to the nucleus. 14 How do the electrons get the required force. Addition of an electron releases energy from the process.
When electrons are removed from an atom that process requires energy to pull the electron away from the nucleus. 13 Why do electrons orbit around the nucleus. With an electron removed the atom possesses a plus one charge therefore it is a positive ion.
More electrons removed -. 15 What happens when electrons get excited. 12 How does changing the number of electrons affect an atom.
Then move towards the electrons. 11 What happens when an electron is added to an atom. If remove all the electrons from the atom so atom gets highly positively charge and it is also possible that all the protons inside that atom repels each other because there is not any negative charge that can make it neutral.
What would happen if we gave an electron enough energy to reach E0. But this stands true only for protium 1H1 which is one among the three isotopes of hydrogen. If we remove an electron from a stable atom the atom becomes electrically incomplete.
A cation positive ion is formed with an electrical charge 1. Electron affinities are negative numbers because energy is released. And this time again atom only contains the mass of its neutron only.
On removing the electron the atom turns into a cation and yes can be used to represent a proton H. 15 Do electrons orbit the nucleus in perfect paths. A hydrogen ion is generated when a hydrogen atom loses an electron and becomes positively charged charge 1.
When a hydrogen ion encounters another substance with an opposite charge it will. What happens when an electron is removed from an atom. 14 What happens to electrons when heated.
Ions are of great interest in science since they are part of plenty of phenomena in Physics Electronics Chemistry. Why does hydrogen become an ion. The energy required to take the electron to E0 is called the ionization energy.
When an electron is not bound by the atom we say that the atom has become ionized. On removing one electron from deuterium it.
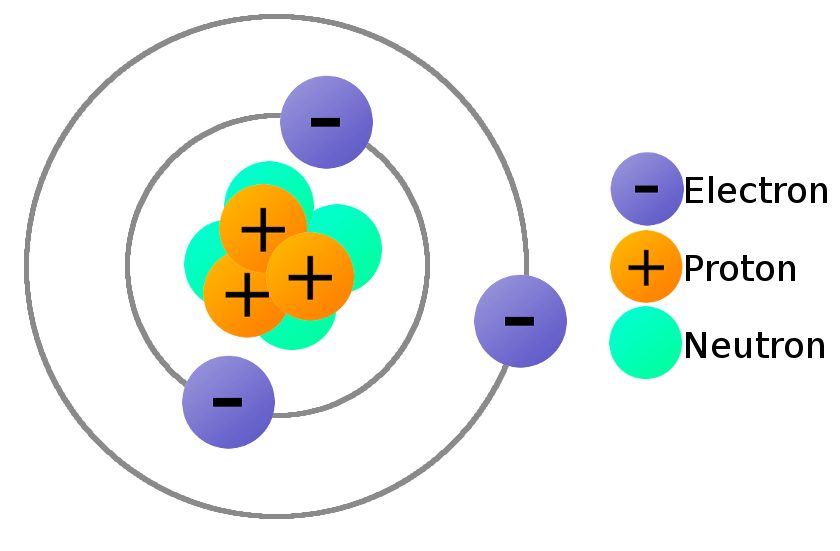
What Is Electricity Learn Sparkfun Com

Electrons Structure Properties Expii

What Is Free Electron And Basic Free Electron Concept Electrical4u
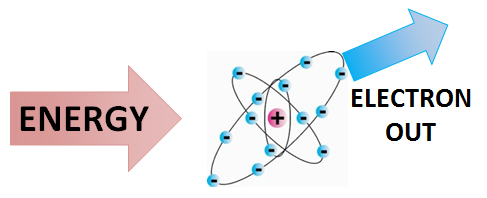
No comments for "What Happens When an Electron Is Removed From an Atom"
Post a Comment